Refractive
July 2023
by Liz Hillman
Editorial Co-Director
Over the last 20 years, refractive surgery has expanded beyond the cornea and to intraocular correction. There have been so many advancements on the diagnostic and procedural front that Y. Ralph Chu, MD, said refractive surgery is truly its own subspecialty.
“It takes equipment commitment, mental commitment. You have to structure your practice around refractive surgery. I think that over the last 10 or more years, refractive surgery has evolved to where unless you’re fully committed to it, it’s hard to just dabble in it,” he said. “You need the right diagnostic technology. You need easy access to laser technology. There is staff education, energy, and internal expertise needed to manage the high expectations of the modern refractive surgery patient.”


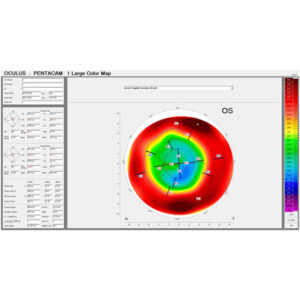
Source: Renato Ambrosio Jr., MD, PhD
Some of the more recent advancements have been at the diagnostic level. Specific to LASIK, Dr. Chu said one of the biggest things to happen on the preoperative screening front is epithelial thickness mapping. This, Dr. Chu said, helps surgeons identify what might be causing asymmetry on topography as it relates to the epithelial surface.
“Sometimes asymmetry on the topography can be the result of epithelial basement membrane dystrophy. It could be the result of epithelial disorders that aren’t structural anomalies, so patients can still have a refractive surgery. Epithelial mapping has helped surgeons screen in and screen out patients who are good or bad patients more effectively,” he said.
Renato Ambrosio Jr., MD, PhD, shared with EyeWorld an editorial on the concept of multimodal refractive imaging1 and a review paper he co-authored describing the value of epithelial thickness mapping. The authors concluded that conscious application of such measurements “significantly impacts safety and efficacy of refractive surgery, and also allows for significant improvement for therapeutic procedures.”2
Dry eye diagnostic technologies and tests have also impacted the screening of patients.
“I think incorporating some type of advanced diagnostic dry eye test is important, and I think for a refractive surgeon, tear breakup time and meibomian gland imaging are helpful because the quality of the tears is important for quality of vision after any refractive surgery,” Dr. Chu said.
If dry eye is identified and able to be addressed preoperatively, Dr. Chu said it might allow a patient to be a candidate for LASIK or another refractive procedure, like SMILE.
In a presentation that Dr. Ambrosio sent to EyeWorld, he credited the range of advances in diagnostics that allow the surgeon to determine the best refractive procedure for each patient’s unique situation, as well as prevent complications in patients who might be at a higher risk. He also said in the presentation that it’s important to remember whether the procedure is refractive or therapeutic.
“We have to understand each patient individually, their individual needs, and the current condition so that we set proper expectations if a patient needs a therapeutic procedure in which we use refractive technology,” Dr. Ambrosio said in the presentation, mentioning an editorial from 2013 in the Brazilian Journal of Ophthalmology.3 “It’s imperative when we talk about the goals of a patient who needs a therapeutic procedure, [it is] to improve the corrected vision. Elective refractive surgery aims to improve uncorrected vision. Properly setting expectations and goals will clearly define what success is.”
From a surgical perspective, Dr. Chu said the biggest advancements have been new surgical procedures becoming available, like SMILE, the EVO ICL (STAAR Surgical), and advanced technology IOLs. Dr. Chu also said that intraoperative guidance systems are a newer piece of equipment that has allowed surgeons to treat astigmatism with lens-based solutions more accurately.
In terms of laser vision correction like LASIK, Dr. Chu said that there have been improvements in ablation profiles over the years that create smoother transitions across the eye. “The smoother the transition, the smoother the ablation, the less regression, the less enhancements, and the more accurate the procedure,” he said.
There’s also been improvements in understanding the importance of residual stromal bed and the impact of percent tissue altered over the years.
“There is some general consensus now that residual stromal bed should be about 300 microns and over for LASIK patients. But it also depends on the percent tissue altered, which means if the residual stromal bed is 300 and over but you did have to take off a significant amount of tissue to achieve the refractive correction, that could be a risk factor for ectasia even though you kept your residual stromal bed above 300,” he explained.
This is where having a comprehensive refractive mindset comes into play. Dr. Chu said if a patient might not be the best candidate for LASIK due to a high refraction and a cornea that’s of low to low-normal thickness, the conversation can shift to other technologies that are now available.
“This is not just LASIK anymore. We think more comprehensively across the five different procedures—PRK, LASIK, SMILE, ICL, and refractive lens exchange—for treating people,” he said. “It’s gotten more exciting, more complex.”
What’s next? Dr. Chu said he’d like to see continued improvement in diagnostic screening for refractive surgery candidacy. He said what he would like are pieces of equipment that integrate multiple tests into one device. He acknowledged some negatives to that (such as if the device breaks) but said it would greatly improve efficiency in the preoperative setting. Procedurally, Dr. Chu said that LASIK already works well, and other options in the market, such as SMILE and ICLs, could continue to see improvements.
In his presentation, Dr. Ambrosio mentioned the convergence of “applied artificial intelligence with ancient philosophy being important for customized treatments and individualized medicine” going forward. He shared with EyeWorld examples of clinical studies on how artificial intelligence can enhance interpretation of Scheimpflug tomography,4 and integrating it with corneal biomechanics,4,5,6 to reduce the risk of ectasia after refractive surgery.
ARTICLE SIDEBAR
Preop diagnostic and screening technologies for refractive surgery
- Slit lamp biomicroscopy
- Central corneal thickness
- Placido topography
- Ocular surface imaging
- 3D Scheimpflug tomography
- Corneal total and internal wavefront imaging
- Ocular scatter imaging
- Ocular biometry
- Segmental tomography
- IOP/corneal biomechanic measurements
- Specular/confocal microscopy
- Osmolarity, molecular biology, and genetic testing
Source: Renato Ambrosio Jr., MD, PhD
About the physicians
Renato Ambrosio Jr., MD, PhD
Professor of Ophthalmology
Federal University of the State of Rio de Janeiro and Federal University of Sao Paulo
Rio de Janeiro, Brazil
Y. Ralph Chu, MD
Founder and Medical Director
Chu Vision Institute
Bloomington, Minnesota
Relevant disclosures
Ambrosio: Alcon, Carl Zeiss Meditec, Mediphacos, Oculus
Chu: Carl Zeiss Meditec
References
- Ambrosio R. Multimodal imaging for refractive surgery: Quo vadis? Indian J Ophthalmol. 2020;68:2647–2649.
- Salomao MQ, et al. Role of the corneal epithelium measurements in keratorefractive surgery. Curr Opin Ophthalmol. 2017;28:326–336.
- Ambrosio R. Therapeutic refractive surgery: why we should differentiate? Rev Bras Oftalmol. 2013;72:85–86.
- Lopes BT, et al. Enhanced tomographic assessment to detect corneal ectasia based on artificial intelligence. Am J Ophthalmol. 2018;195:223–232.
- Ambrosio R, et al. Integration of Scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg. 2017;33:434–443.
- Ambrosio R, et al. Optimized artificial intelligence for enhanced ectasia detection using Scheimpflug-based corneal tomography and biomechanical data. Am J Ophthalmol. 2022;251:126–142.
Contact
Ambrosio: dr.renatoambrosio@gmail.com
Chu: yrchu@chuvision.com