Cornea: OP-ED
Spring 2024
by Edward Holland, MD, and Albert Cheung, MD
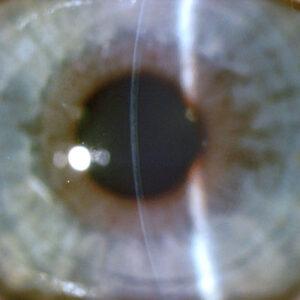
Despite breakthroughs in our ability to manage severe ocular surface disease (OSD) with limbal stem cell deficiency (LSCD) effectively both medically and surgically, the majority of patients with this condition are either not treated or are not treated appropriately. Safe and effective treatment options with Ocular Surface Stem Cell Transplantation with Systemic Immunosuppression (OSSTx with SI) have been developed and presented at major meetings, in peer-reviewed journals as well as textbooks. Techniques and protocols for OSSTx with SI have been published for more than 25 years.1–6 Despite this, only a few corneal specialists have adopted these treatments, and many of these patients are not referred to the few centers that perform these procedures. Misdiagnosis and mismanagement of these patients are sadly all too common. This has led to the inappropriate application of penetrating keratoplasty or keratoprosthesis, often worsening an already bad situation.
While it is difficult to assess the exact prevalence of LSCD, approximately 1–5 per 10,000 has been estimated.7 Given the U.S. population of greater than 335 million, that would affect approximately 33,500 to 167,500 people.
Too often, LSCD patients are only medically managed based on a diagnosis of dry eye disease or viral keratitis. When they are managed surgically, substandard surgeries are commonly offered. Superficial keratectomy (with or without amniotic membrane) and phototherapeutic keratectomy can buy time with only short-term visual improvement for mild disease with anterior stromal scarring.8 Ultimately, this strategy will be unsuccessful, as it fails to address the underlying problem of limbal stem cell failure.
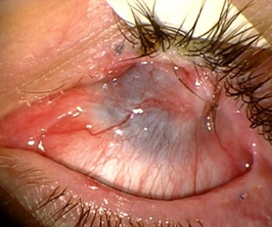
Penetrating keratoplasty (PK) is the most common erroneously applied corneal procedure we encounter for total LSCD patients.8 Corneal specialists should be well aware that there is no chance of long-term success with a primary PK for these patients.1,8–10 The total LSCD leads to a failed PK due to ocular surface failure, inflammation, and neovascularization of the cornea with subsequent rejection and worse vision.8,11
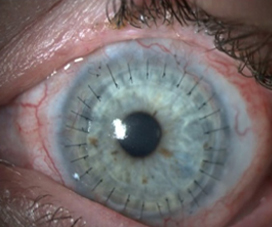
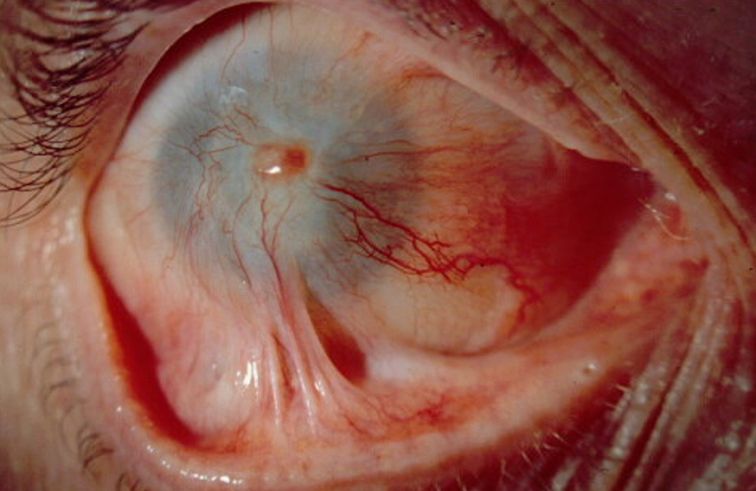
Many corneal specialists perform the Boston keratoprosthesis (KPro) for LSCD. Overall, KPros have a reasonable success rate in non-inflamed eyes, such as failed PKs or high-risk PKs due to immunologic reasons. However, KPro for LSCD is a different story. Studies have noted worse KPro outcomes in the setting of LSCD and OSD. In a large multicenter cohort of Boston type 1 KPro eyes, Srikumaran et al. noted the following rates of sterile corneal necrosis (19.5%), retinal detachment (18.6%), endophthalmitis (15.5%), and infectious keratitis (without endophthalmitis, 3.4%) for all KPro eyes.12 They noted that in eyes with severe OSD (defined as severe keratoconjunctivitis sicca, LSCD, and cicatrizing conjunctivitis) had significantly lower retention rates (35% at 84 months) compared to eyes without OSD (78% at 84 months). Aravena et al. found that eyes undergoing a KPro implantation for failed keratoplasty had a retention failure rate of 0.052/year while eyes with SJS, chemical injury, and MMP had retention failure rates of 0.299/year, 0.094/year, and 0.147/year.13
We have published our poor KPro results for total LSCD patients: 2.4% developed endophthalmitis with devastating visual consequences (vision decreased to light perception in 2 of 3 eyes), and 16% encountered melt-related complications. In addition, 47% of patients lost vision due to glaucoma in just 32 months followup.14 Due to our poor results, we stopped offering and performing primary KPros in this patient population unless there is a contraindication for OSSTx.
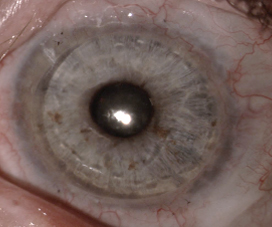
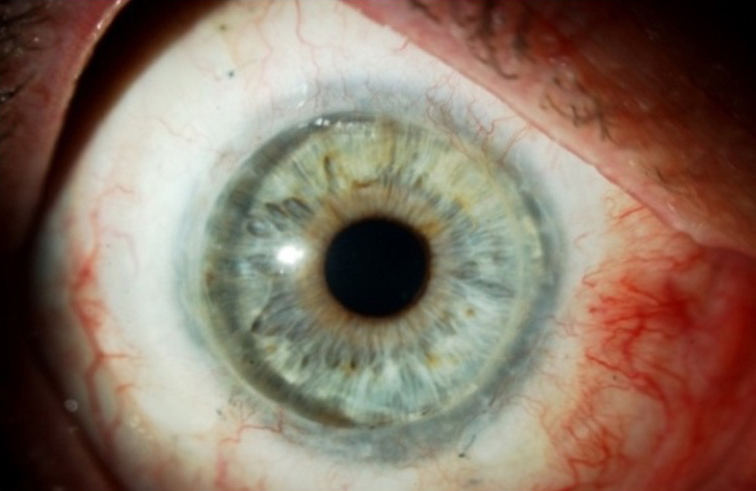
Obstacles in adoption of OSSTx with SI
There are many reasons why corneal surgeons have not embraced OSSTx with SI. First, corneal specialists fear managing or don’t want to manage systemic immunosuppression, despite the fact that a safe and acceptable treatment with immunosuppression is well published and demonstrates long-term efficacy of maintaining a stable ocular surface.3-5 This fear on the part of the clinician is often communicated to the patient. There appears to be a misunderstanding by corneal surgeons regarding the safety of SI even with appropriate monitoring. Corneal surgeons may not be aware of the literature supporting the safety of this approach or feel comfortable enough to follow these protocols.
Cornea is the only transplant specialty that does not employ the principles of systemic immunosuppression, which are not needed for routine keratoplasty but are beneficial in high-risk keratoplasty (i.e., prior graft rejection, stromal neovascularization) in addition to OSSTx.15 We have used SI on more than 1,000 patients and have seen minimal severe adverse events (rare cardiovascular disease and one secondary malignancy) and no deaths. We have published on our use of these medications and the protocol to adopt the principles of SI from organ transplantation experts, notably nephrology (Cincinnati protocol).3,5 Partnering with renal transplant specialists on preoperative screening, tissue typing of donors, SI level monitoring, and management of medication side effects has provided a treatment paradigm for corneal surgeons to use SI safely and effectively to achieve excellent outcomes for our patients.
Source (all): Edward Holland, MD
Recommendations
In our goal to achieve better outcomes in patients with severe OSD with LSCD, we want to encourage our cornea colleagues to recognize this issue at an individual, corneal service, departmental, and societal level. From our experience, LSCD is the leading cause of untreated corneal blindness in the U.S.
It has been more than 30 years since the techniques of OSSTx with SI have been introduced. The surgical techniques have improved, valuable preoperative screening has been applied, and the SI medications are safer and more effective. Yet we have not seen a significant change in corneal surgeons’ behavior. Given the small number of clinicians performing these procedures, we need to acknowledge the scope and severity of this problem. These patients have a terrible quality of life due to blindness and chronic ocular pain. As a community, we must do better and offer safe and effective OSSTx.
We do not expect every corneal surgeon to perform OSSTx with SI. However, we think major ophthalmology centers should build programs, and groups of corneal surgeons should collaborate to create regional centers to make this treatment more accessible to help this population with the biggest unmet need in our field.
About the authors
Albert Cheung, MD
Virginia Eye Consultants
Norfolk, Virginia
Edward Holland, MD
Professor of Ophthalmology
University of Cincinnati
Cincinnati, Ohio
References
- Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677–743.
- Croasdale CR, et al. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52–58.
- Holland EJ, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31:655–661.
- Movahedan A, et al. Long-term outcomes of ocular surface stem cell allograft transplantation. Am J Ophthalmol. 2017;184:97–107.
- Cheung AY, et al. Cincinnati protocol for preoperative screening and donor selection for ocular surface stem cell transplantation. Cornea. 2018;37:1192–1197.
- Cheung AY, et al. Clinical outcomes of allogeneic ocular surface stem cell transplantation in pediatric patients. Cornea. 2021;40:54–60.
- Orphanet. Limbal stem cell deficiency. www.orpha.net. Accessed October 15, 2022.
- Cheung AY, et al. Limbal stem cell deficiency: demographics and clinical characteristics of a large retrospective series at a single tertiary referral center. Cornea. 2021;40:1525–1531.
- Tugal-Tutkun I, et al. Penetrating keratoplasty in cicatrizing conjunctival diseases. Ophthalmology. 1995;102:576–585.
- Sepsakos L, et al. Outcomes of keratoplasty after ocular surface stem cell transplantation. Cornea. 2017;36:1025–1030.
- Sangwan VS. Limbal stem cells in health and disease. Biosci Rep. 2001;21:385–405.
- Srikumaran D, et al. Long-term outcomes of Boston type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology. 2014;121:2159–2164.
- Aravena C, Yu F, Aldave AJ. Long-term visual outcomes, complications, and retention of the Boston type I keratoprosthesis. Cornea 2018;37:3-10.
- Chan CC, et al. Incidence, risk factors, and surgical management of Boston type 1 keratoprothesis corneal melts, leaks, and extrusions. Cornea. 2016;35:1049–1056
- Azam AM, et al. Immunosuppressive therapy for high-risk corneal transplant. Curr Ophthalmol Rep. 2022; 10:114–129.
Relevant disclosures
Cheung: None
Holland: None
Contact
Cheung: acheung@cvphealth.com
Holland: eholland@holprovision.com