International
January 2018
by Claudio Orlich, MD
ALACCSA shares a recent article on spherical aberrations from one of its contributors
Today the goal of cataract surgery is to provide patients with the best possible quality of vision. While it is common to see patients post-cataract surgery with a visual acuity (VA) of 20/20, many patients remain dissatisfied with their quality of vision. This is due to several factors, including problems with the ocular surface, pseudophakic dysphotopsia, and optical aberrations in general.
Although low order aberrations (myopia, hyperopia, and regular astigmatism) have a greater impact on vision, high order aberrations also play an important role, especially in patients who are candidates for multifocal lenses. Among the high order aberrations that can be corrected with cataract surgery is spherical aberration. There are multiple intraocular lens options that can correct this aberration, but when and how we should use them is a topic of debate and research in recent years.
The word “aspheric” is used to describe a surface or in this case a lens that does not have a spherical shape. In an aspherical lens, the rays of light passing through the center do not focus at the same point as the rays passing through the periphery of the lens.

Naturally, the human cornea is aspheric, usually a bit more curved in the center and flattened toward the periphery; we call this form “prolate.” In a prolated cornea, the rays of light that cross the center of the cornea tend to converge or focus on a point anterior to the peripheral rays, and this aberration is called “negative spherical aberration.” In an oblate cornea, the central light rays are focused behind the peripheral rays, and this aberration is called “positive spherical aberration” (Figure 1).
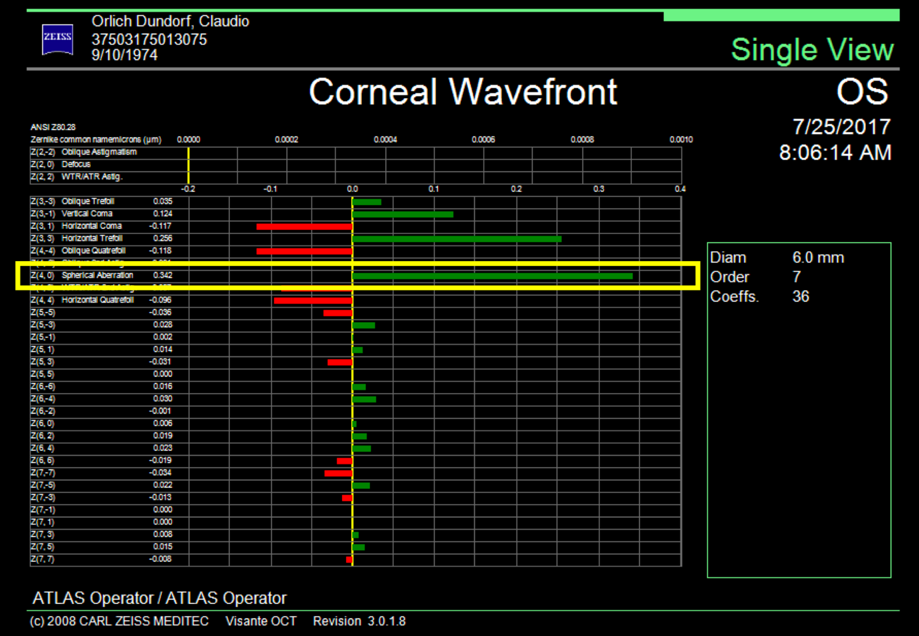
Spherical aberration is included within the high order aberrations, specifically in the group of fourth order aberrations, along with quatrefoil and secondary astigmatism. Spherical aberration generally reduces retinal image contrast and affects visual quality, especially under mesopic conditions.1
The average spherical aberration of the anterior cornea surface is slightly positive (between +0.27 and +0.30 μm), remaining stable throughout life.2 The natural crystalline compensates for this positive spherical aberration, inducing a negative spherical aberration of –0.20 μm, leaving a slightly positive total aberration of +0.10 μm. Spherical aberration of the lens changes over time unlike spherical aberration of the cornea, going from negative to positive as cataracts develop. This positive spherical aberration of the cataractous lens adds to the spherical positive aberration of the cornea, impairing the visual quality of patients. Based on this concept, intraocular lenses were developed with negative spherical aberration, which simulate a young lens that compensates for the average positive spherical aberration of the cornea. Some studies suggest that it is not necessary to correct spherical aberration completely, and in fact it is recommended to leave a slightly positive residual (+0.10 μm). A study performed on pilots of the American Air Force by Grimson et al. suggests that a quantity of positive spherical aberration can be correlated with visual acuities of 20/15 or better.3 It is impossible to completely correct the spherical aberration in all our patients as there is an interaction between much more complex aberrations than a sum of the existing spherical aberration and the intraocular lens induced aberration. Nevertheless, the objective must be directed to a final low spherical aberration, which allows the patient good contrast sensitivity.
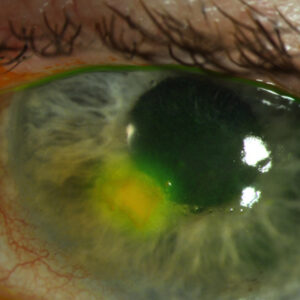
In the consultation, we will find that the corneal spherical aberration varies greatly among individuals, especially in the presence of pathological corneas or modified ones by post-refractive surgery. In a myopic treatment with excimer laser, a central flattening of the cornea is induced, generating an oblate cornea, more flat in the center than in the periphery, inducing a positive spherical aberration. In contrast, a hypermetropic correction increases the central curvature of the anterior surface of the cornea, generating a hyperprolate cornea, inducing greater negative spherical aberration that may contraindicate the use of an aspheric intraocular lens, which instead of correcting would worsen the existing spherical negative aberration, deteriorating the quality of vision.
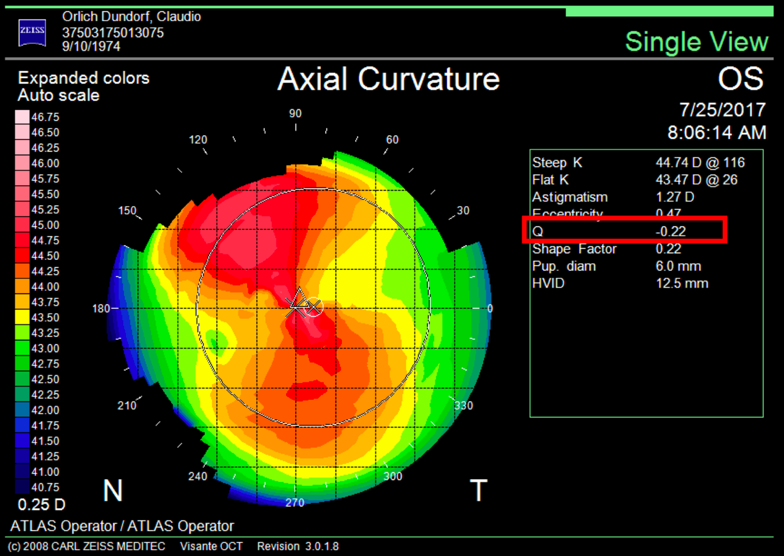
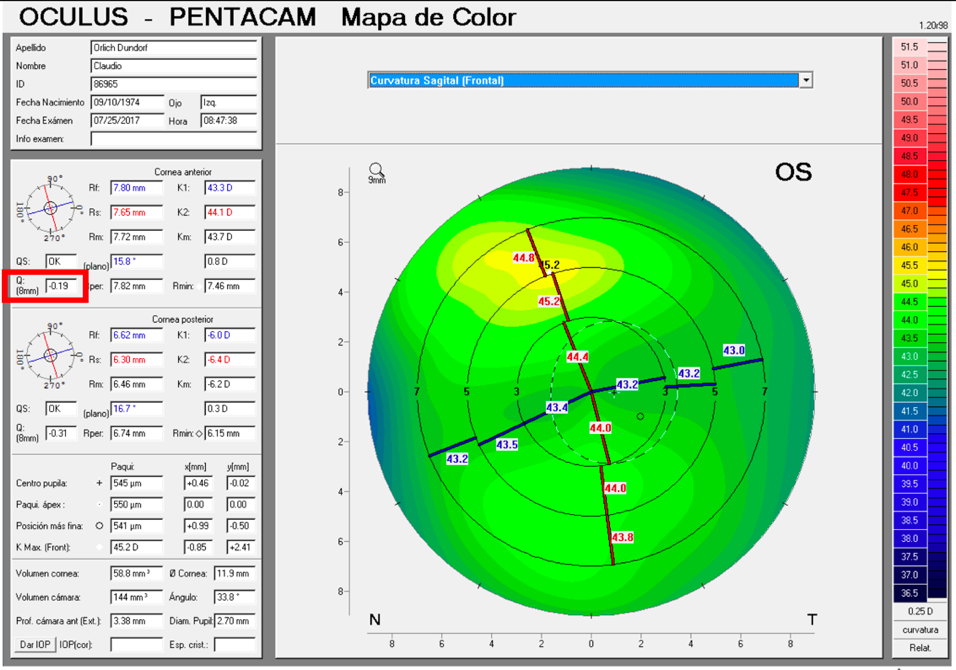
It is important to differentiate spherical aberration from corneal asphericity or commonly named with the Q coefficient. Both are related but different. Asphericity (Q) is a factor that tells us how much and how the cornea is peripherally flattened from the corneal apex, that is, if the cornea is prolate or oblate, while informing us of the degree of asphericity. A cornea with a Q of –0.20 is not the same as a cornea with a Q of –0.45; in both cases the cornea is prolate, but the prolaticity of the second case is greater, which means that the surface has a radius of peripheral curvature more flat than the first, and this means that it will have greater negative spherical aberration. A normal aspheric cornea has a Q factor between –0.20 and –0.45; a Q of zero would correspond to a completely spherical cornea, and a Q greater than zero corresponds to an oblate cornea, i.e., more powerful at the periphery than in the center, inducing positive spherical aberration. A hyperprolate cornea is considered to have a Q factor >0.6. Any corneal topographer nowadays will give us the Q value (Figure 2).
Intraocular lenses can induce positive spherical aberration, be neutral or induce negative spherical aberration.
Lenses that induce positive spherical aberration
Spherical intraocular lenses increase spherical positive aberration in the eye, reducing the quality of the retinal image in most patients. There are few cases in which these lenses would be prescribed, such as in patients with hyperprolate corneas (operated with hypermetropic excimer laser). Among the lenses with positive spherical aberration are the MA60AT (Alcon, Fort Worth, Texas) with a spherical aberration of +0.14 +/–0.09 μm, the CT Spheris 204 (Carl Zeiss Meditec, Jena, Germany), and the Sensar lens (Johnson & Johnson Vision, Santa Ana, California).
Lenses that do not modify spherical aberration
The neutral lenses currently available on the market with prolate anterior and posterior surfaces are the Akreos, SofPort, L161 (Bausch +Lomb, Bridgewater, New Jersey), and the CT Asphina 409M (Carl Zeiss Meditec). These do not modify corneal spherical aberration, are less sensitive to tilt than aspherical lenses, and provide better image quality than spherical IOLs.
Lenses that induce negative spherical aberration
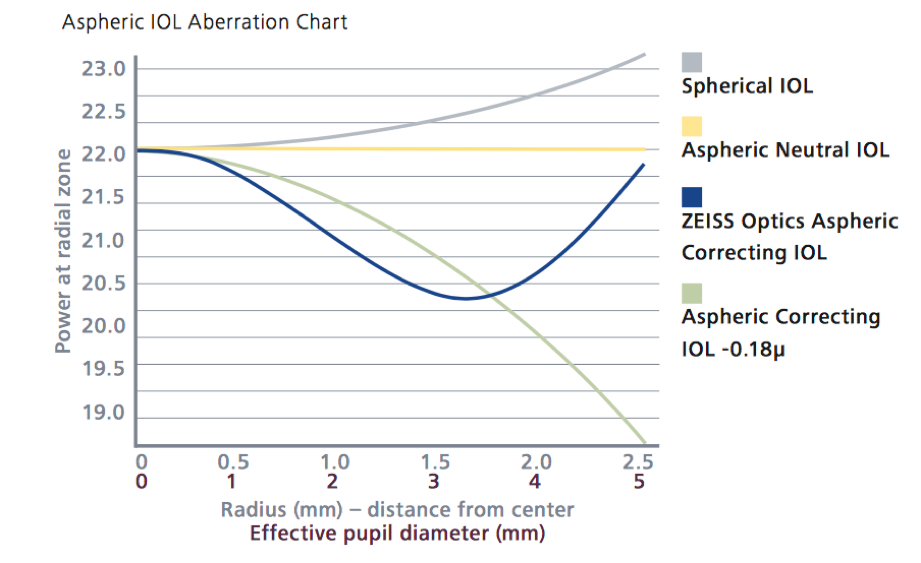
Some aspherical lenses have been designed with a prolate anterior surface (Tecnis, Johnson & Johnson Vision), a prolate posterior surface (AcrySof IQ, Alcon), or with both prolate surfaces (FineVision, PhysIOL, Liège, Belgium, and CT Asphina 509M). These give better contrast sensitivity when correcting positive spherical aberration of the cornea but generate less depth of focus than spherical lenses (Figure 4). In addition, they require a better centering, since a decentration of the optics of the lens induces other aberrations like coma. The performance of the lens depends on the pupil, and its function deteriorates in mesopic conditions.
There are lenses with aspherical profiles that tolerate a greater lens offset, such as the CT LUCIA lens (Carl Zeiss Meditec). The “aspheric Zeiss optics” has a different design known as bi-sign,4 in which the IOL combines the advantages of neutral and correcting aspherical IOLs, making this lens a good choice for most patients and ideal for cases with an alpha angle greater than 0.5 mm or cases with the risk of the lens off center (Figure 5).
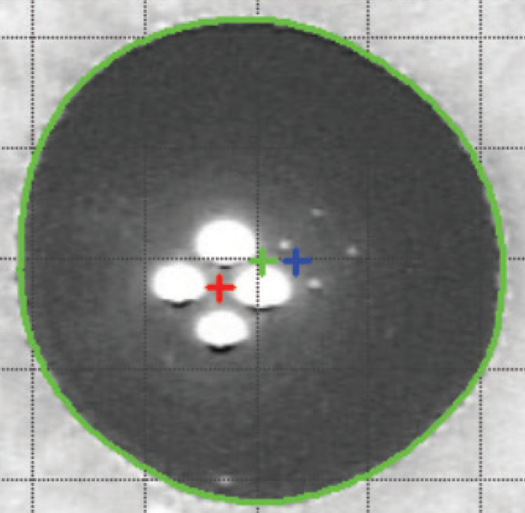
The alpha angle, unlike the kappa angle (best known for its importance in refractive surgery), is the distance between the center of the limbus and the visual axis. Under normal conditions an intraocular lens will be centered within the capsular bag, and the center of the bag will be very close to the center of the limbus. The alpha angle can be measured with the Pentacam (Oculus, Wetzlar, Germany) or iTrace (Tracey Technologies, Houston) (Figure 6).
Some authors do not recommend implanting multifocal intraocular lenses in patients with alpha angles greater than 0.5 mm.5 The iTrace device classifies with colors the amount of alpha angle; an angle less than or equal to 0.3 mm is green, 0.3 to 0.5 mm is yellow, and greater than 0.5 mm is red. A green alpha angle provides greater confidence that the patient will be looking through the center of the optic zone, as intended. One may consider implanting some multifocal lenses when the alpha angle is in the yellow zone. When there is an alpha angle in the red zone (>0.5 mm), the optical axis (center of the capsular bag) may not coincide with the visual axis of the patient, which may lead to a refractive surprise or an unsatisfied patient. This same principle used for multifocal lenses could be used for aspheric lenses.
Some colleagues have proposed selecting the intraocular lens platform depending on the corneal asphericity to correct; others prefer to leave a residual positive spherical aberration to give greater depth of focus to patients. In patients with multifocal lenses, with special corneal or previous refractive surgery, spherical aberration becomes more important. It is well known that multifocal lenses distribute the light in several foci, which contributes to losing between 8 and 20% of the transmitted. All these lenses present a loss of contrast at different distances; it is at this point that it is important to make use of all the resources that can improve contrast sensitivity to give greater visual quality to this group of patients. For the cataract surgeon, it is important to know the corneal asphericity of patients to offer them the best possible result.
Source (all): Claudio Orlich, MD
Concepts to remember
- In general, correction of spherical aberration improves contrast sensitivity but decreases depth of focus.
- The spherical aberration is greater as the pupil size increases.
- For proper correction of corneal spherical aberration, the intraocular lens should be perfectly centered.
- In view of the possibility of intraocular lens deviation, it is preferable to use lenses with neutral spherical aberration or IOLs with bi-sign design.
- Hyperprolate corneas (Q >0.6) do not use aspherical lenses.
References
1. Oshika T, et al. Influence of pupil diameter on the relation between ocular higher-order aberration and contrast sensitivity after laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2006;47:1334–8.
2. Guirao A. Optical aberrations of the human cornea as a function of age. J Opt Soc Am A Opt Image Sci Vis. 2000;17:1697–702.
3. Grimson JM, et al. Contrast sensitivity: establishing normative data for use in screening prospective naval pilots. Aviat Space Environ Med. 2002;73:28–35.
4. Portney V. New bi-sign aspheric IOL and its application. Optom Vis Sci. 2012;89:80–9.
5. Piracha AR. Using angle alpha in premium IOL screening. Cataract & Refractive Surgery Today. 2016;(3):24–25.
Editors’ note
This article was first published in ALACCSA-R #24, September/October 2017, pages 10–15, and is included here with permission from ALACCSA. For more information about ALACCSA, visit www.alaccsa.com.
Contact information
Orlich: orlichclaudio@hotmail.com