Cornea
December 2020
by Ellen Stodola
Editorial Co-Director
There are several companies in the field of artificial irises. Kevin M. Miller, MD, and Sathish Srinivasan, MD, discussed the available products and important considerations when working with artificial irises.
Currently, there is only one product FDA approved in the U.S., the CUSTOMFLEX (HumanOptics), which has models with and without fiber. Other companies working on such devices that are not yet commercially available in the U.S. are Morcher and Reper. Dr. Miller said Ophtec was working on artificial irises but as of January 2020 discontinued sale. Dr. Miller has experience with the Morcher artificial iris under an investigational device exemption, making him the only person in the U.S. allowed to implant it.
Dr. Miller performs 1–3 artificial iris cases a month. In general, he said these cases are a lot of work from start to finish.
Dr. Srinivasan has a few more options with the CE mark available to him in the U.K. He has experience with the Ophtec, Morcher, and HumanOptics devices and said he has done more than 100 cases. Most are patients with large iris defects, he said, noting that they’re not for cosmetic iris color changes.
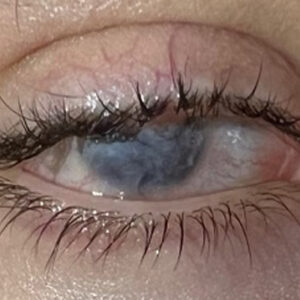
Source: Kevin M. Miller, MD
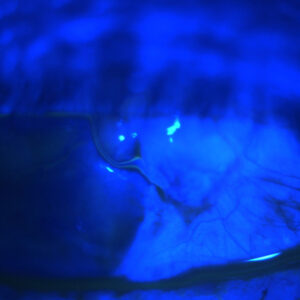
Source: Kevin M. Miller, MD
Best patients for artificial irises
Dr. Srinivasan again stressed that artificial irises are for medical use, such as for patients who have had trauma or damage to the iris or pupil for which sutures cannot be used. A partial or total artificial iris to reconstruct the pupil, iris, or both may be necessary, he said.
The treatment plan depends on the size of the defect. “Some of the defects could be corrected using a cosmetic contact lens with an artificial iris or pupil painted on it,” Dr. Srinivasan said. “If that doesn’t work, then we have to see how big the defect is.” A partial defect might accommodate surgical iris reconstruction, but if there is extensive iris or pupil loss, that’s where artificial devices come into play, he said.
Morcher makes partial implants that can help with smaller defects and is the only company to do so. Larger defects merit a full artificial iris. The Morcher product, Dr. Srinivasan said, is not custom made, so this can be used if it fits the patient’s profile. The HumanOptics artificial iris is a custom implant based on a photograph of the fellow eye (assuming the fellow eye is normal). The Reper device is also off the shelf, so the sizing is not custom, but the color can be customized.
Dr. Srinivasan highlighted the learning curve with these devices and said “it requires a lot of skill.” Physicians have to go through training and get certified before the companies will allow them to buy an implant and use it. There are a number of training courses at the annual meetings of ASCRS, AAO, and ESCRS that can help surgeons become familiar with these devices.
Dr. Srinivasan mentioned several contraindications. An artificial iris is not for patients who have a natural lens inside the eye. It would need to be combined with a phacoemulsification procedure or used in patients who are already pseudophakic. Dr. Miller said the devices are not implanted in congenital aniridics who have clear lenses.
Dr. Miller said artificial irises can be used for anyone who has a large iris defect that can’t be fixed with sutures. This is a good option for these patients with large defects because very few patients can tolerate other options for the problem, which include closing the eye, patching the eye, wearing tinted glasses, or wearing a thick contact lens.
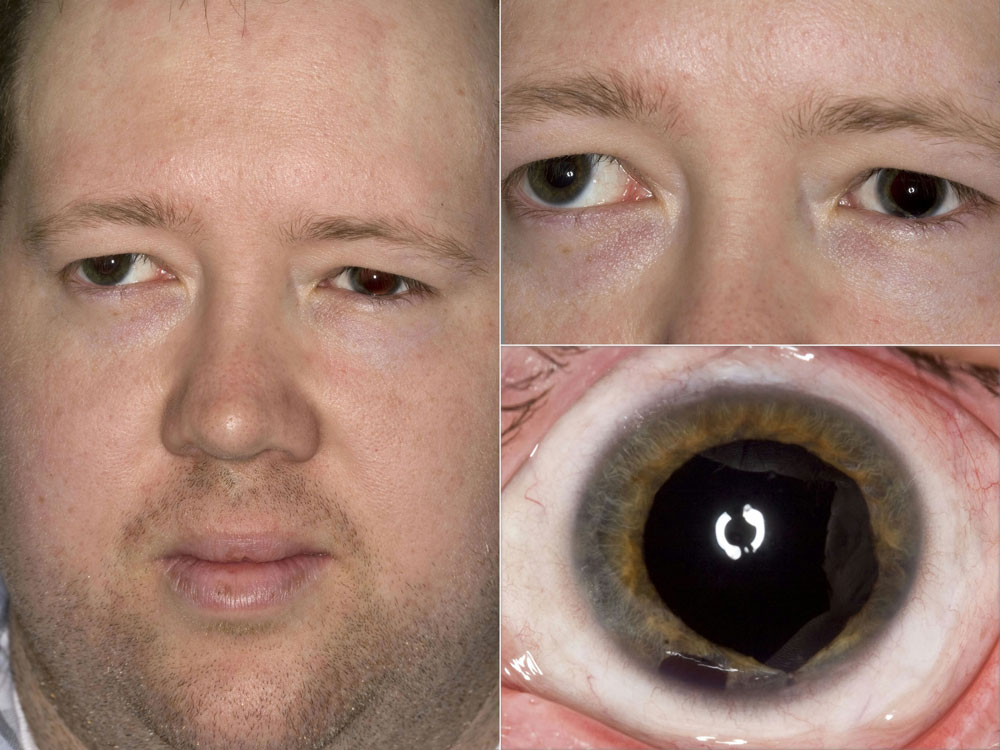
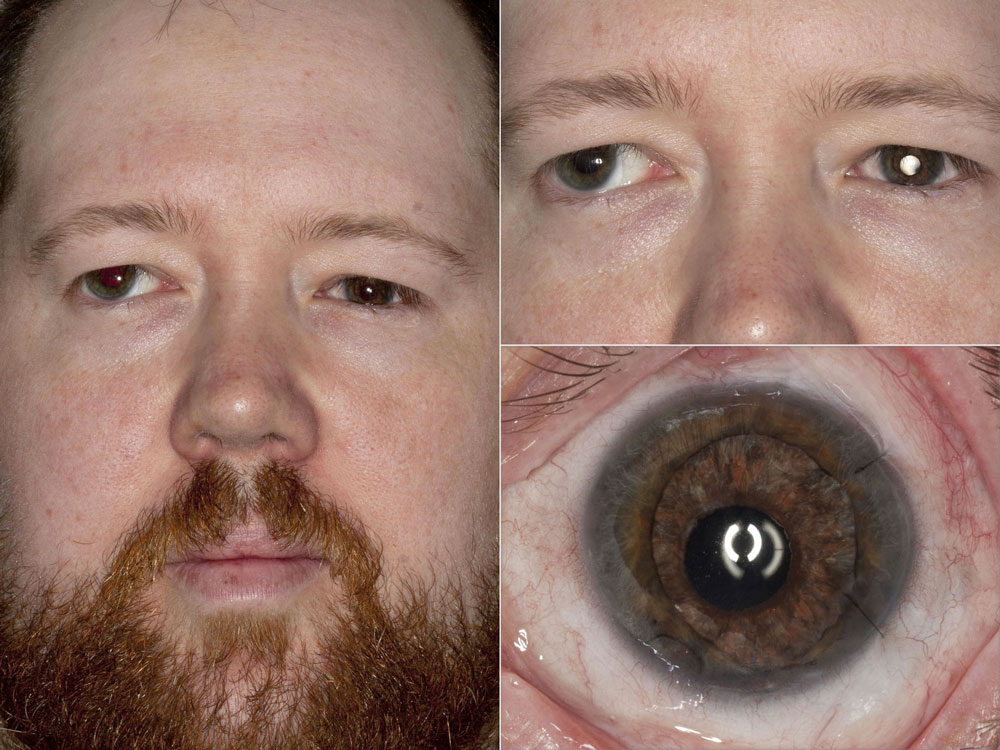
From a cosmetic standpoint, Dr. Miller favors the HumanOptics product. To make the iris, you take a picture of the patient’s good eye, which the company then uses to hand paint the artificial iris. Dr. Miller noted that this FDA-approved device does not have a lens implant at this point, but devices from other companies do have a built-in optic in the pupil. He said this would be a nice advancement in the future.
At this point, Dr. Miller said the primary limitation for artificial irises is the price. Another limitation is the process for matching the color of the eye. Because the iris is created from a picture sent to the company, lighting is critical. If the eye has major scarring, Dr. Miller said you may be able to notice the difference once the artificial iris is in, but for the most part, it’s hard to tell at a conversational distance. If there’s residual iris tissue in the eye, that may be darker than the implant as well.
Dr. Miller said centration is another factor to be concerned about. When you’re suturing, especially with an open sky configuration, you don’t know for sure if it will be centered when the cornea is back on. If it’s not centered, it will be too late at that point, Dr. Miller said. This would be most noticeable in people with blue irises.
In terms of pearls for placing the device, Dr. Miller said that every patient is unique, and there’s no cookie-cutter approach. “There’s so much pathology in these patients that surgeons who do this have to have a lot of tricks in their toolbox,” he said.
Dr. Miller said that patients who receive these devices are often very happy with the outcome. “The vision is one thing, but there’s a huge psychological component when you lose a part of your facial anatomy that’s important for self-esteem and cosmesis,” he said.
Reimbursement
With FDA approval still being relatively new, Dr. Miller said reimbursement is difficult in that it’s inconsistent.
Medicare will cover the artificial iris, but the device has to be ordered prior to surgery and paid for first. It’s then covered after the surgery is complete. Being around $8,100 out of pocket ahead of time, this can be a challenge for patients.
Dr. Miller noted that there are three CPT codes associated with artificial irises: one for the artificial iris going in, one for the artificial iris plus cataract surgery, and one for an iris/lens exchange. Almost all of these patients are getting other surgeries as well, he said, and those can be billed in addition to the base CPT code.
Dr. Srinivasan also mentioned the cost factor in the U.S.; in the U.K., the artificial iris is covered. “We have to go through special permissions,” he said. This involves filling out extra paperwork to justify its use, but he said that the price of the device has been covered for all of his patients.
About the physicians
Kevin M. Miller, MD
Kolokotrones Chair in Ophthalmology
David Geffen School of Medicine at UCLA
Los Angeles, California
Sathish Srinivasan, MD
University Hospital Ayr
Ayr, U.K.
Relevant disclosures
Miller: None
Srinivasan: None
Contact
Miller: kmiller@ucla.edu
Srinivasan: sathish.srinivasan@gmail.com